Foreign Property News | Posted by Aye Myat Thu
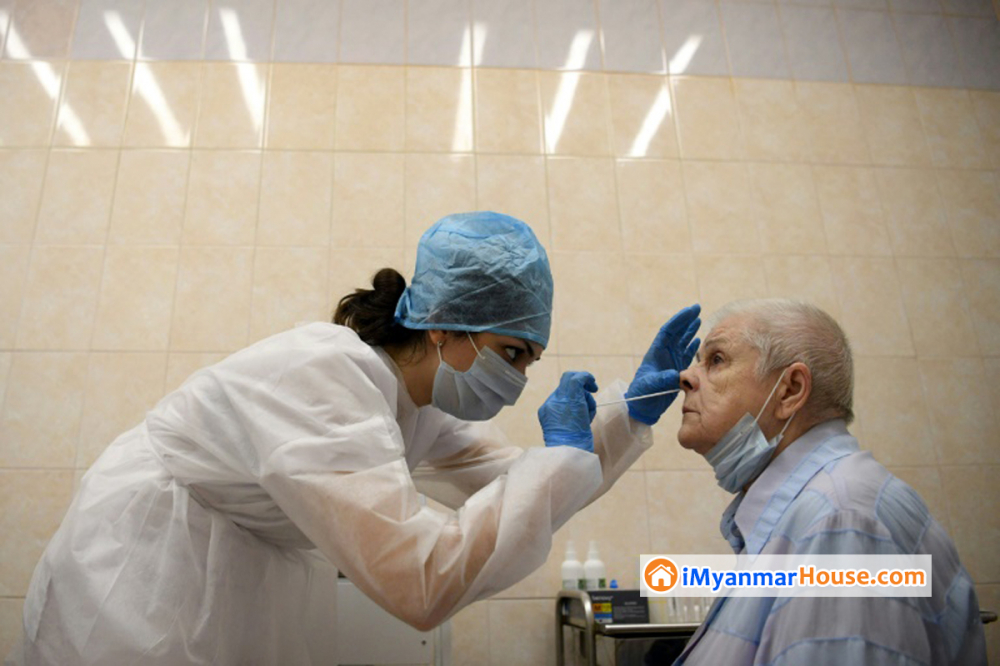
The mayor of Moscow invited residents Wednesday to partake in a new coronavirus vaccination trial reportedly approved by the national government earlier this month.
The international medical community has voiced concern over Russia’s hastened approval of a vaccine without it first being tested in large-scale advanced trials, involving tens of thousands of people. Scientists further point to the lack of shared data as a break in medical community standards.
But Moscow Mayor Sergei Sobyanin has suggested that some form of testing will be conducted, saying the “post-registration research” will have a six-month duration and involve 40,000 people.
“We all were eager to see the creation of a vaccine, and now we have it,” Sobyanin said. “Now, Moscow residents have a unique chance to become the main participants in a clinical research that will help defeat the coronavirus.”
Sobyanin said the vaccine was proven to be safe on previous research.
“The post-registration clinical trial will allow for a permanent registration certificate and expansion of the circle of possible vaccine recipients, including the 60+ age group,” the Russian Direct Investment Fund said.
The fund, which has bankrolled the development of the vaccine, also reportedly said additional trials will be conducted in five other countries, thought they did not specify which.
Russian President Vladimir Putin boasted the effectiveness of the vaccine earlier this month by saying that his two adult daughters have been inoculated. Putin claimed the vaccine underwent adequate testing and showed lasting immunity to the virus – though he has not provided any data proving the effectiveness of the vaccine.
Ref: foxnews









