The coronavirus vaccine developed by Chinese company Sinovac is up to 97% effective, according to interim data from tests by Indonesia’s state-owned pharmaceutical company Bio Farma. “Our clinical trial team found, within one month, that the interim data shows up to 97% for its efficacy,” Bio Farma spokesperson Iwan Setiawan said at a news conference, reports Reuters. Some 1,600 people participated in the clinical trials. However, the exact efficacy of the vaccine will not be determined until January, according to a Sinovac spokesperson, as these are interim data, as the company is still gathering data on efficacy from the ongoing Phase 3 trial. According to him, the...
View Details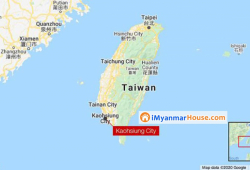
Taiwanese authorities have slapped a $3,500 fine on a man who broke quarantine regulations for just eight seconds. The man, a migrant worker from the Philippines, was quarantining in a hotel in Kaohsiung City when he briefly stepped out of his room into the hallway, the city's Department of Health told Taiwan's official Central News Agency (CNA). The man was caught on CCTV by hotel staff, who contacted the Department of Health, CNA reported. The department fined him 100,000 Taiwan dollars -- around $3,500. Under Taiwan's quarantine rules people are not allowed to leave their rooms, no matter for how long. People in quarantine should not think they won't be fined for leaving their hotel...
View Details
Turkey and Brazil are among the countries hard hit with coronavirus infections. Both are moving forward with plans to use an unproven COVID-19 vaccine developed by China’s Sinovac Biotech. The vaccine, called CoronaVac, is currently carrying out its Phase 3 trials. Turkish Health Minister Fahrettin Koca announced a vaccine plan that includes the CoronaVac. The country has an agreement with Sinovac for 50 million shots. The first shipment of the vaccines will arrive in Turkey after December 11. Koca said early use approval would be given to the vaccine after Turkish laboratories confirm its safety and after early results from Phase 3 trials are examined. “If developments...
View Details
Homegrown pharma major Bharat Biotech has sought approval from Drug Controller General of India (DCGI) for the emergency use authorisation (EUA) of its COVID-19 vaccine, Covaxin. This is India's indigenous coronavirus vaccine developed by Hyderabad-based Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) - National Institute of Virology (NIV). This is the third request received by the DCGI in the last two few days. American drug maker Pfizer and Pune-based Serum Institute of India have filed for EUA of their respective vaccine candidates. Covaxin is an inactivated vaccine undergoing Phase 3 clinical trials with 26,000 participants in over 25 centres across...
View Details
One person has died and at least 292 have been hospitalized with symptoms resembling an epilepsy attack in India's southeastern state of Andhra Pradesh on Sunday as India media sounds the alarm about a new "mysterious disease." With the hotbed reportedly in the Eluru city, the disease caused infected individuals to suddenly pass out after suffering symptoms including shivering, frothing at the mouth and nausea. As reported by Press Trust of India, the deceased was a 45-year-old man who was admitted to the Government General Hospital in Eluru on Sunday morning and died in the evening. Most of the hospitalized individuals were reportedly let go after undergoing necessary tests and an...
View Details
Indonesia’s government said 1.2 million doses of a COVID-19 vaccine developed by China-based biopharmaceutical company Sinovac Biotech arrived in the country late Sunday. President Joko Widodo said in a televised address that another 1.8 million doses of the vaccine are expected to arrive in early January. “We are very grateful, thank God, the vaccine is now available so that we can immediately curb the spread of the COVID-19 disease,” Widodo said. The government is still waiting for millions of other doses of the Sinovac vaccine to arrive in the form of raw materials that will be further processed by state-owned pharmaceutical holding company PT Bio...
View Details
Bats in the frontier regions of south and south-west China harbour other coronaviruses that already have the capacity to cross over to humans, a prominent Chinese scientist has said. Dr Shi Zhengli of the Wuhan Institute of Virology said these viruses, including close relatives of Sars-CoV-2, which causes Covid-19, were likely to be circulating in nature beyond China.“We should not only search for them in China, but also in south Asian countries,” said Shi, who is nicknamed “Bat Woman” because her research group studies bat coronaviruses. Her comments, which were made at a webinar organised by the French medical and veterinary academies, came as two...
View Details
The number of people in the U.S. killed by Covid-19 could nearly double in the next several months despite a nationwide vaccine rollout, health researchers warn. The U.S. is forecast to see a cumulative 539,000 deaths by April 1, according to a Dec. 4 report published by the Institute for Health Metrics and Evaluation at the University of Washington’s School of Medicine. More than 279,000 people in the U.S. have died from the virus and more than 14.3 million have been infected, according to data from Johns Hopkins University. The country is expected to approve and begin distributing one or more vaccines as early as December. IHME researchers forecast that the expected vaccine...
View Details
Britain's Queen Elizabeth II will receive the Pfizer-BioNTech coronavirus vaccine within weeks, after UK regulators granted emergency approval and the world's first roll-out begins next week, reports late Saturday said. The monarch, 94, and her 99-year-old husband Prince Philip are in line to get the jab early due to their age and will not receive preferential treatment, the Mail on Sunday reported. The newspaper said Britain's most senior royals would reveal they have been given the inoculation "to encourage more people to take up the vital jab", amid fears so-called anti-vaxxers could dent enthusiasm for it. Britain on Wednesday gave emergency approval to the Pfizer-BioNTech...
View Details
The Texas doctor captured in a photo cradling a distraught Covid-19 patient on Thanksgiving has spoken of the sad isolation of his elderly patients and pleaded with people to "do the basic things" to avoid infection and stay out of hospital. Hospitalizations from Covid-19 reached another record high across the US Monday, with officials across several states expressing concern that health care facilities would be overwhelmed. Dr. Joseph Varon, chief of staff at United Memorial Medical Center in Houston, told CNN's New Day on Monday that he had worked for 256 days nonstop in the pandemic so far and was frustrated by the increasing numbers of people being hospitalized. Varon described the...
View DetailsCopyright © 2025 iMyanmarHouse.com | All Rights Reserved.